Up the Walls of the Worlds
(after James Tiptree’s novel)
[Click to embiggen image]
The flood of data from the solar system probes has led to tectonic shifts in our understanding of our neighbors. High on that list has been the discovery of liquid water in the large moons of the gas giants: Jupiter’s Europa and Ganymede, Saturn’s Enceladus. By many strands of evidence – which include geysers – all three have large subsurface salt-water seas below their icy surfaces (Saturn’s Titan has surface oceans but they’re made of methane and ethane, liquid at Titan’s ambient temperature). In Europa and Enceladus this water may be in contact with a complex silicate core that could supply both directional scaffolding and building blocks; and the orbital friction between the moons and their primaries generates heat dynamos that could give rise to deep hydrothermal vents.
Salt water with a steady supply of dissolved nutrients; an energy source; complex silicates that – according to Cairns-Smith’s clay hypothesis – could act as anchors and template propagators for chiral organic molecules. The simultaneous presence of all these components is a recipe for the development of life.
Life as we know it is based on carbon and uses water as its solvent. Both are unique within their respective categories. Carbon is the best foundational element for complex chemistry by several orders of magnitude: the number and variety of organic compounds exceeds that of all the rest combined. Like its fellow occupants of the fourth column of the periodic table, carbon has an outermost electron orbital that is exactly half-occupied. So the fourth column elements are equally good as electron donors or acceptors, and as a result they can form compounds with just about every other element.
Carbon has an additional almost-unique characteristic: its unoccupied orbital is at such a distance from the nucleus that it can form bonds of the exactly correct strength to create very large and complex compounds. In particular, carbon bonds with itself more or less with the same proclivity that it bonds with anything else. Whatever can be imagined, of any size, shape, taste or smell, can be found among organic compounds, from diamonds to nucleic acids, from limonenes to fullerenes. If a carbon atom’s four available positions have distinct occupants, the resulting compounds are chiral (“handed”), another apparent prerequisite for biomolecules – certainly a decisive attribute of all terrestrial ones.
Silicon, the next foundational candidate after carbon, is a distinctly inferior also-ran. The radius of its outer electron shell is larger, which means that it forms weaker bonds, especially with itself. Generally compounds with more than three silicon atoms in a row are very unstable, unless they are forced into a crystal lattice. And if oxygen is anywhere near it, all available silicon funnels itself into silicates, precluding all other combinations.
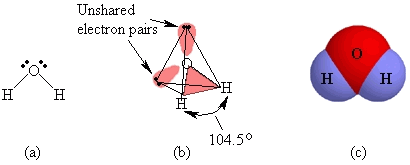
In turn, water has several properties that make it the most potent and versatile solvent. This includes its tetrahedral structure (due to its two free electron pairs, which make it a polar compound), phenomenal heat capacity (which makes it a stabilizer), high heat conductivity and surface tension (vital for many cellular structures and processes), transparency to visible light (crucial for photosynthesis) and the anomalous property of becoming less dense when it solidifies into ice (hence a reservoir and refuge). The runner-up, ammonia, shares some of these attributes (including the tetrahedral structure) and might be the solvent of choice at the lower temperatures of Titan’s hydrocarbon seas. Such a configuration is fully deployed in Joan Vinge’s justly famous Eyes of Amber.
If we encounter life elsewhere, it’s a foregone conclusion that its details will differ significantly from ours – and that its differences will dwarf what SF has come up with. Non-terrestrial life may not use DNA or RNA as its basis of genetic transmission; it may use a different kit of starting blocks for energy, scaffolding and catalysis. It will have a totally different repertoire of body plans, sensoria, mental processes, reproductive modes, ecosystems. But it will be based on carbon and will almost certainly use water as its solvent. And just from current percentages, it’s possible that most planetary life may have developed in “roofed ocean” worlds like Europa, instead of the open atmosphere of Earth.
Walking rightward (as is customary) in the Drake equation, the question is: if they emerge, would roofed-ocean lifeforms evolve to complexity? To sentience? To use of technology, whereby they might send the unambiguously civilizational signals still eagerly awaited by SETI? Of course, our horizons are limited by our own intrinsic parochialisms. We cannot easily visualize technology that’s not based on metals and fire. We cannot easily imagine how sentients that never see the stars might nevertheless deduce their existence.
Many of these perceived hurdles are in fact easily overcome. In Forerunner Foray, André Norton postulated a species that directed the building activity of coral polyps. The solution of water-dwelling lifeforms would be direct-to-biotech, bypassing metal forges. Terrestrial cephalopods are remarkably intelligent and are known to use technology (cetaceans are revenants to water, so their intelligence springs from the same foundation as ours). As for guessing the existence of the stars, a species with sensors in the right bracket of the EM spectrum would rapidly become aware of the overwhelming nearby presence of Jupiter or Saturn. Such species might eventually build starships from tissue, like Farscape’s Moya. Beyond that, the specifics of such species might go a long way towards explaining the over-invoked Fermi Paradox: if they sent signals, they would automatically choose their own waterhole frequency.
So far, we’ve seen the exteriors of roofed-ocean worlds. Missions have been planned for investigations of interiors, though their launch dates keep slipping further into the future. In the end, our own vaunted ability to see the stars may not avail us if we choose to turn inward, eventually running out of the metals and fuels that keep our window to the universe open. If we do send out exploratory vessels, we have to be extra careful not to mar the worlds we touch, that may harbor their own tinkerers and dreamers. But it’s my fond fantasy that, before my own life sets, we get to hear of filigree manta rays swirling under Europa’s ice to the radio pulse beats of the giant overhead.
Images: Top, the Europa system (NASA/JPL); middle, water, the marvelous solvent; bottom, a manta ray pirouetting in its element (Getty Images).




Wonderful entry–I appreciate the discussion of water as solvent of choice (and the second-choice ammonia) and carbon as the building block of choice (and why silicon is a poor substitute), along with your mention of stories that use these elements.
It’s fun to think of alien tech, alien ways of thought.
Designing aliens is stupendous fun — but if we ever find any, I suspect our imagination will look stunted compared to reality!
best thing today (besides the kids making me lunch): the word “embiggen”!
The term has become legit, what can I say!
I’m sure you’re right about our imagination seeming stunted compared with the real thing (would love for that day to arrive)
Me, too… I’ll even settle for microbes!
While carbon chemistry and water solvent might be common, I do wonder if oxygen is terribly common. A paper from late last year by Sara Seager, William Baines and colleagues suggests hydrogen might be more common an active gas in atmospheres. It certainly opens up the ecosphere by allowing greenhouse warming of surface oceans out to at least 10 AU around sun-like stars. What do you think Athena? Are O2 breathers an anomaly?
Spectrometric measurements indicate that oxygen is the most abundant element after hydrogen and helium in the universe, slightly ahead of carbon. Please note that I don’t discuss oxygen breathing because I don’t consider it a universal prerequisite, as I do carbon and water.
This just in:
http://www.jpl.nasa.gov/news/news.php?release=2015-166&rn=news.xml&rst=4586
I still say if we want to know if life exists under the ice crust of Europa, all we have to do is land in one of those cracks and examine the rust–colored material in them.
Larry, agreed. There should be at minimum organic material in the cracks and geyser ejecta, even if large items would probably be in shreds.
Great post! Thinking of the processes involved in building starships from tissue is indeed fun, as much so as envisioning alien life forms and civilizations.
There was a flood (pardon the pun!) of recent data, and this topic is always near and dear to my head and heart! So I opined…
I too hope to see a subsurface Europa probe in my lifetime- who knows what we might discover!
I imagine that a starship made of tissue would require a tough shell surrounding the living parts, lest meteoroids and vacuum harm it. Not to mention radiation. Perhaps ice would do. And what would the propulsion system be like?
But perhaps the builders of such a beast would find it as difficult to imagine constructing a spaceship with a non-living technology.
Water is one of the best insulators there is; so aquatic spacefaring species might have an advantage! Such a starship need not be sentient itself. Propulsion might be on the slow side (which would be yet another explanation for the Fermi paradox).
Water supports you against G-forces as well- aquatic aliens could withstand higher g-force than we can.
But propulsion would probably be slow. I was imagining that the aqua-aliens might find it easier to build chemical explosive pulse drives than our kind of rockets. Not as much trouble with cooling- just a reaction plate and some kind of explosive. Advanced biotech should be able to produce chemical explosives, correct? That might get them off their planet/moon, at least.
Probably they would set their course for a Europa-like world rather than Earth, as well!
If they had achieved spacefaring capacity, they’d probably have figured out that alternative biochemistries are possible (just extrapolating from our own species).
True, I had the same thought. But, if they were looking for a home as well, they may have the same bias towards roofed ocean worlds as we do for quasi-Earths as destinations.
It appears that Sol system has both terrestrial and roofed-ocean habitats, so this is a false dilemma as far as our neighborhood is concerned!
Freeman Dyson said we should also check for Europan life in orbit:
http://www.theatlantic.com/past/docs/issues/97nov/space.htm
As for an actual lander on Europa:
http://blog.chron.com/sciguy/2015/05/a-europa-lander-is-possible-jpl-scientists-say-and-congress-appears-likely-to-support-it/
http://spaceflightnow.com/2015/04/10/nasa-invites-esa-to-build-europa-piggyback-probe/
If we are going to send an aquatic species into deep space, I nominate the manatee:
http://io9.com/why-do-manatees-need-space-suits-1679085554
Wouldn’t you rather have them representing Earth life to the rest of the galaxy rather than those smelly, violent, oversexed primates?
Manatees are neat for sure!